Introduction
Sugammadex is a unique reversal agent for aminosteroid-induced neuromuscular blockade.
| Year | Milestone |
|---|---|
| 2000s | Recognition of the need for a more effective reversal agent for neuromuscular blockade. |
| 2001 | Initiation of clinical trials for sugammadex (then known as Org 25969). |
| 2008 | Approval for clinical use in Europe and Australia; marketed as Bridion by Merck & Co. |
| 2010 | Introduction of sugammadex in Japan, becoming the first Asian country to approve its use. |
| 2013 | Approval for use in Canada, expanding its reach to North America. |
| 2015 | Rejection of licensing approval by the U.S. FDA, citing hypersensitivity concerns. |
| 2018 | FDA approval for use in the United States, allowing sugammadex to be used in American medical practices. |
| Present Day | Sugammadex widely used globally as an integral part of anesthesia and neuromuscular blockade reversal, enhancing patient safety and recovery. |
Mechanism of Action
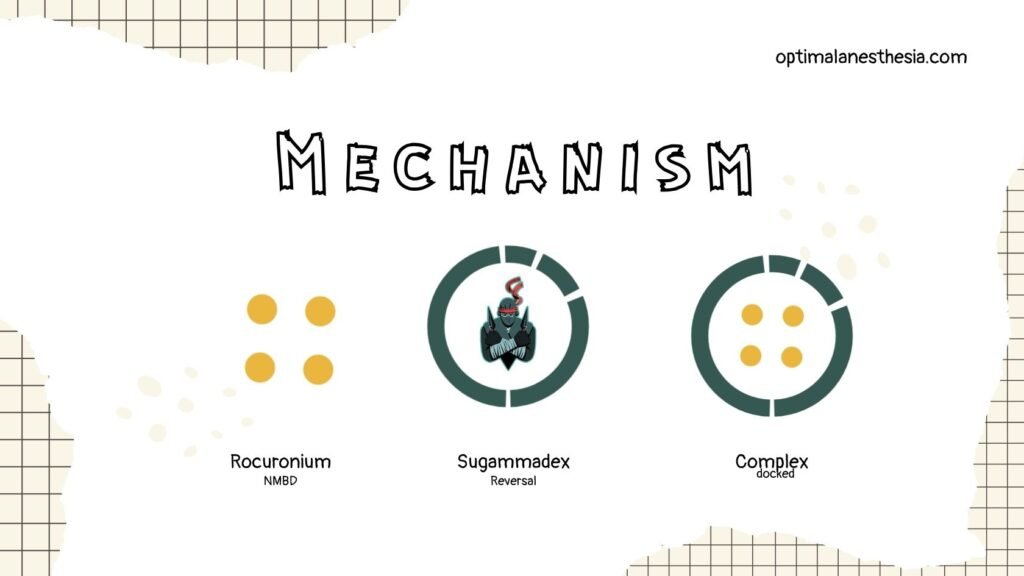
Sugammadex is a direct reversal agent for aminosteroid neuromuscular blocking drugs (NMBD). It encapsulates aminosteroid NMBDs, with the highest affinity for rocuronium, rendering them inactive. It also promotes the dissociation of the aminosteroid from the neuromuscular junction, allowing the return of neuromuscular function (NMF).
Pharmacokinetics
Sugammadex exhibits linear pharmacokinetics in the dose range of 0.1mg/kg to 32mg/kg, with some evidence suggesting a linear relationship up to 96mg/kg. It has an estimated volume of distribution of 11-14 liters, an elimination half-life of approximately two hours, and is primarily excreted unchanged through the kidneys. Notably, sugammadex alters the pharmacokinetics of rocuronium when bound as the rocuronium-sugammadex complex.
Dosing
The dose of sugammadex required depends on the depth of rocuronium-induced neuromuscular blockade. Different depths of block (routine, moderate, profound) are defined based on neuromuscular monitoring. Here is a table showing the dose of sugammadex required for different levels of rocuronium neuromuscular blockade:
| Type of Block | Dose of Sugammadex | Time to TOF >0.9 |
|---|---|---|
| Routine | 2mg/kg | 2 minutes |
| Moderate | 4mg/kg | 3 minutes |
| Profound | 16mg/kg | 1.5 minutes |
For reversal of routine and moderate rocuronium and vecuronium-induced blockade, similar doses of sugammadex are required. However, the dose of sugammadex for profound vecuronium-induced block has not been formally determined.
If repeat neuromuscular blockade is required after sugammadex administration, it is recommended to use an alternative NMBD, although it is possible to re-paralyze the patient with rocuronium based on the sugammadex dose given and the time since administration.
Implications for Practice
Sugammadex is a superior alternative to neostigmine for reversing neuromuscular blockade, providing faster and more reliable reversal. It allows for the use of rocuronium in rapid sequence induction (RSI) and has potential benefits in various clinical scenarios, including electroconvulsive therapy (ECT).
Sugammadex vs. Suxamethonium
Sugammadex, in combination with rocuronium, can emulate the intubating conditions of suxamethonium for RSI, although it has a longer duration of action. The use of sugammadex in ECT has been associated with shorter recovery times and reduced post-operative complaints compared to suxamethonium.
Sugammadex for Rocuronium-Induced Anaphylaxis
Sugammadex has been considered in the treatment of rocuronium-induced anaphylaxis, but its effectiveness is not supported by extensive evidence. Case reports and studies have yielded mixed results.
Specific Populations
- Elderly: Sugammadex can be used in elderly patients, but onset of action may be slower.
- Paediatrics: Sugammadex is recommended for reversing TOF count 2 in children aged two to 17, but not endorsed for neonates or infants.
- Pregnancy and Breastfeeding: Its use during pregnancy is not recommended, but there have been case reports of post-caesarean section administration.
- Obesity: There is debate about dosing in obese patients, but dosing based on ideal body weight plus 40% appears safe.
Specific Considerations
- Neurological Disease: Sugammadex is effective in patients with myasthenia gravis and other neurological conditions.
- Cardiorespiratory Disease: It is generally safe for patients with pulmonary and cardiac conditions.
- Renal Disease: Sugammadex use is not recommended in patients with creatinine clearance <30ml/min, but some studies suggest it may still be effective.
- Liver Disease: Further research is needed for patients with hepatic dysfunction.
Drug Interactions
Sugammadex should not be co-administered with ondansetron, verapamil, and ranitidine due to physical incompatibility. It may have capturing and displacement reactions with specific drugs, requiring caution.
Adverse Effects
The most concerning adverse effect is hypersensitivity, though rare. Other common side effects include dysgeusia, headache, nausea, vomiting, and urticaria. It is generally well-tolerated, but precautions should be taken in specific cases.


